Potassium hydrogen phthalate is a solid, monoprotic acid frequently used in the laboratory to standardize strong base solutions. It has the unwieldy formula of KHC8H4O4. This is often written in shorthand notation as K How many grams of KHP are needed?
1 Answer
Jun 9, 2018
Well, let us look at the structure of
Explanation:
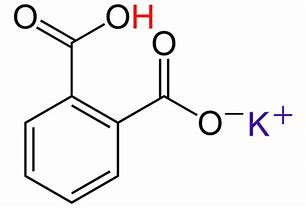
And clearly this can react with ONE EQUIV of base....
And ALSO with ONE equiv of ACID....
And because the phthalate salt is crystalline, relatively high molecular mass, and not terribly hygroscopic, this is an excellent primary standard for acid-base titrations...
But you have delineated NEITHER the concentration of the acid or base you use, NOR the mass of the phthalate salt...so we cannot address your question..

