Predict whether the change in entropy, for the following reaction will be positive or negative H2(g) + (1/2) O2(g) → H2O(g) . Why is this positive, even though there are less mols in the product?
1 Answer
Mar 31, 2018
Because you got lied to?
By pretending that these are ideal gases (in addition, that they are spherical particles), we would predict
#"H"_2(g) + 1/2"O"_2(g) -> "H"_2"O"(g)#
Here are the entropies:
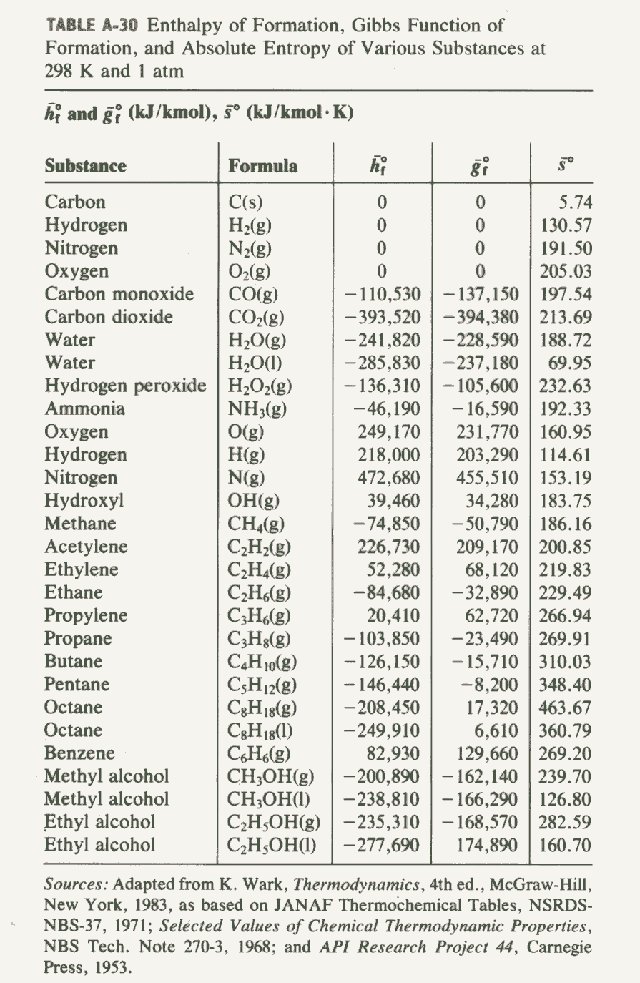
And thus,
#color(blue)(DeltaS_(rxn)^@) = sum_P S_P^@ - sum_R S_R^@#
#= "1 mol" cdot "188.72 J/mol"cdot"K" - ["1 mol" cdot "130.57 J/mol"cdot"K" + 1/2 "mols" cdot "205.03 J/mol"cdot"K"]#
#= color(blue)(-"44.37 J/K")#

