Question dealing with pseudo-first order reaction?
I really only want the answer to part a), but I'd appreciate the rest as well.
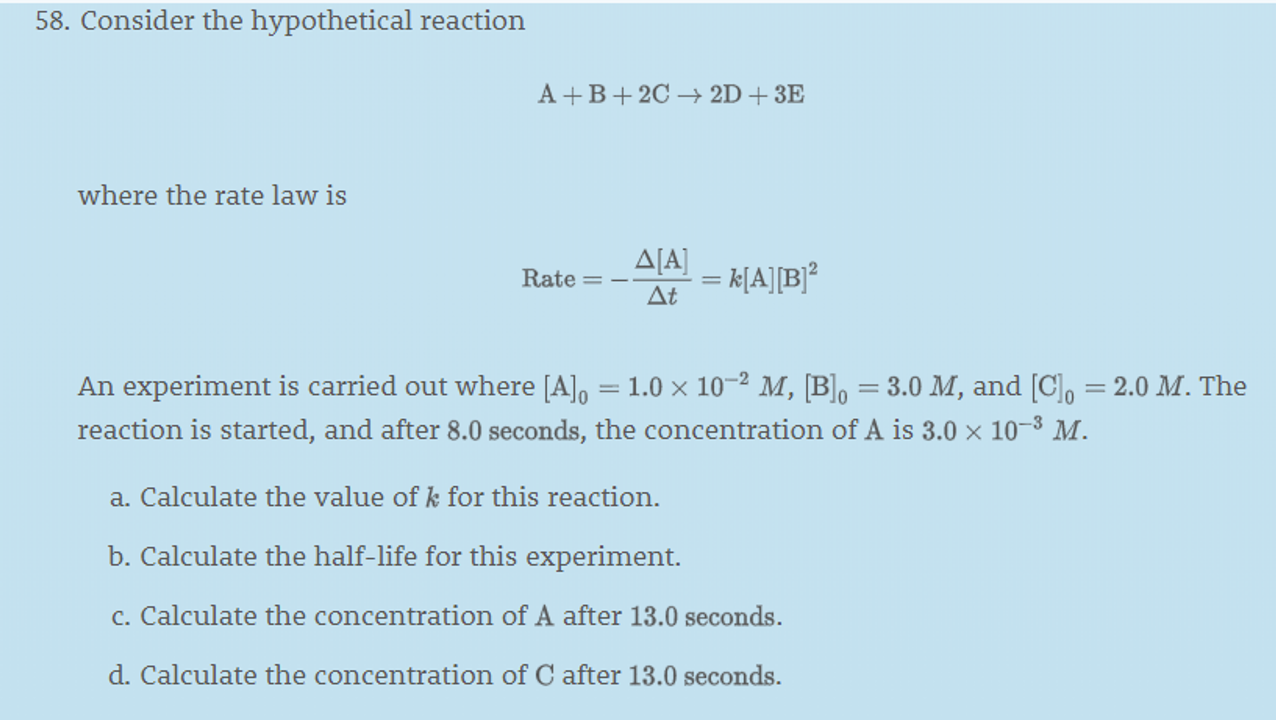
I really only want the answer to part a), but I'd appreciate the rest as well.

1 Answer
You only know that
#C# is zero order, but that#B# is second order. Therefore, you do not assume that#Delta[B] = 0# , and you still don't assume that#Delta[C] = 0# either (only that#C# does not affect the rate).However, we see that
#[B]# and#[C]# are about#100# times larger than#[A]# , so we assume that the reaction is pseudo-first order with respect to#A# , i.e. that
#Delta[B]# #"<<"# #Delta[A]#
#Delta[C]# #"<<"# #Delta[A]# Therefore, we can use the first-order integrated rate law for one reactant to get
#k'# :
#ln[A] = -k't + ln[A]_0# where
#t# is the current time,#[A]# is concentration of#A# in#"M"# , and#[A]_0# is the starting concentration of#A# .Solving for
#k'# ,
#ln(([A])/([A]_0)) = -k't# .This gives
#color(blue)(k') = 1/tln(([A]_0)/([A]))#
#= 1/("8 s") ln((1.0 xx 10^(-2) "M")/(3.0 xx 10^(-3) "M"))#
#= color(blue)("0.15 s"^(-1))#
Since the reaction is supposed to be pseudo-first order,
#r(t) = k[A][B]^2~~ k'[A]#
What we actually got was
#color(blue)(k) ~~ (k')/([B]^2) ~~ ("0.15 s"^(-1))/("3.0 M")^2#
#= color(blue)("0.0167 M"^(-2)cdot"s"^(-1))#
for the overall reaction.
So then, as before, we assume that this is pseudo-first order with respect to
#A# , giving a pseudo-first order half-life based on#A# .
#ln(1/2[A]_0) = -kt_(1//2) + ln[A]_0#
#ln((1/2[A]_0)/([A]_0)) = -kt_(1//2)#
#ln2 = kt_(1//2)# Therefore, the half-life is:
#color(blue)(t_(1//2)) = (ln2)/k#
#= (ln2)/("0.15 s"^(-1)) = color(blue)("4.6 s")#
This can again be done using the first-order integrated rate law.
#ln[A] = -kt + ln[A]_0#
#color(blue)([A]) = [A]_0e^(-kt)#
#= 1.0 xx 10^(-2) "M" cdot e^(-"0.15 s"^(-1) cdot "13.0 s")#
#= color(blue)(1.4 xx 10^(-3) "M")# Does this make physical sense? Well, it took longer than
#8# seconds, and this concentration is indeed less than#3.0 xx 10^(-3) "M"# accomplished after#8# seconds.
For
#C# , consider the stoichiometry of the problem. You will consume twice as much#C# as you do#A# .(The reaction is zero order with respect to
#C# though, so keep that in mind. If we didn't have#Delta[A]# , we wouldn't have any idea how to get#[C]# at any time#t# because#C# doesn't influence the rate.)After
#"13.0 s"# ,#A# decreased by:
#|Delta[A]| = |1.4 xx 10^(-3) "M" - 1.0 xx 10^(-2) "M"|#
#= 8.6 xx 10^(-3) "M"#
#C# would decrease by twice as much, so
#|Delta[C]| = 2(8.6 xx 10^(-3) "M") = 1.7 xx 10^(-2) "M"# Therefore,
#color(blue)([C]_(13)) = [C]_0 - |Delta[C]_(0->13)|#
#= "2.0 M" - 1.7 xx 10^(-2) "M"#
#=# #"1.98 M"#
#~~# #color(blue)("2.0 M")# to two sig figs.(It was only a
#0.86%# reduction in#[C]# .)This proves that the assumption for a pseudo-first order reaction is good.

