Rank from longest to shortest wavelength?
Place the following transitions of the hydrogen atom in order from longest to shortest wavelength of the photon emitted.
n=7 to n=4
n=5 to n=3
n=4 to n=2
n=3 to n=2
Place the following transitions of the hydrogen atom in order from longest to shortest wavelength of the photon emitted.
n=7 to n=4
n=5 to n=3
n=4 to n=2
n=3 to n=2
1 Answer
Explanation:
The electrons that fall to the (
The electrons that fall to the (
The electrons that fall to the (
wavelengths (> 1400 nm).
I have indicated the transitions in the diagram below.
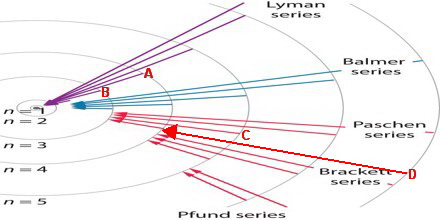
(Adapted from Assignment Point)
In order of decreasing wavelength, the lines are

