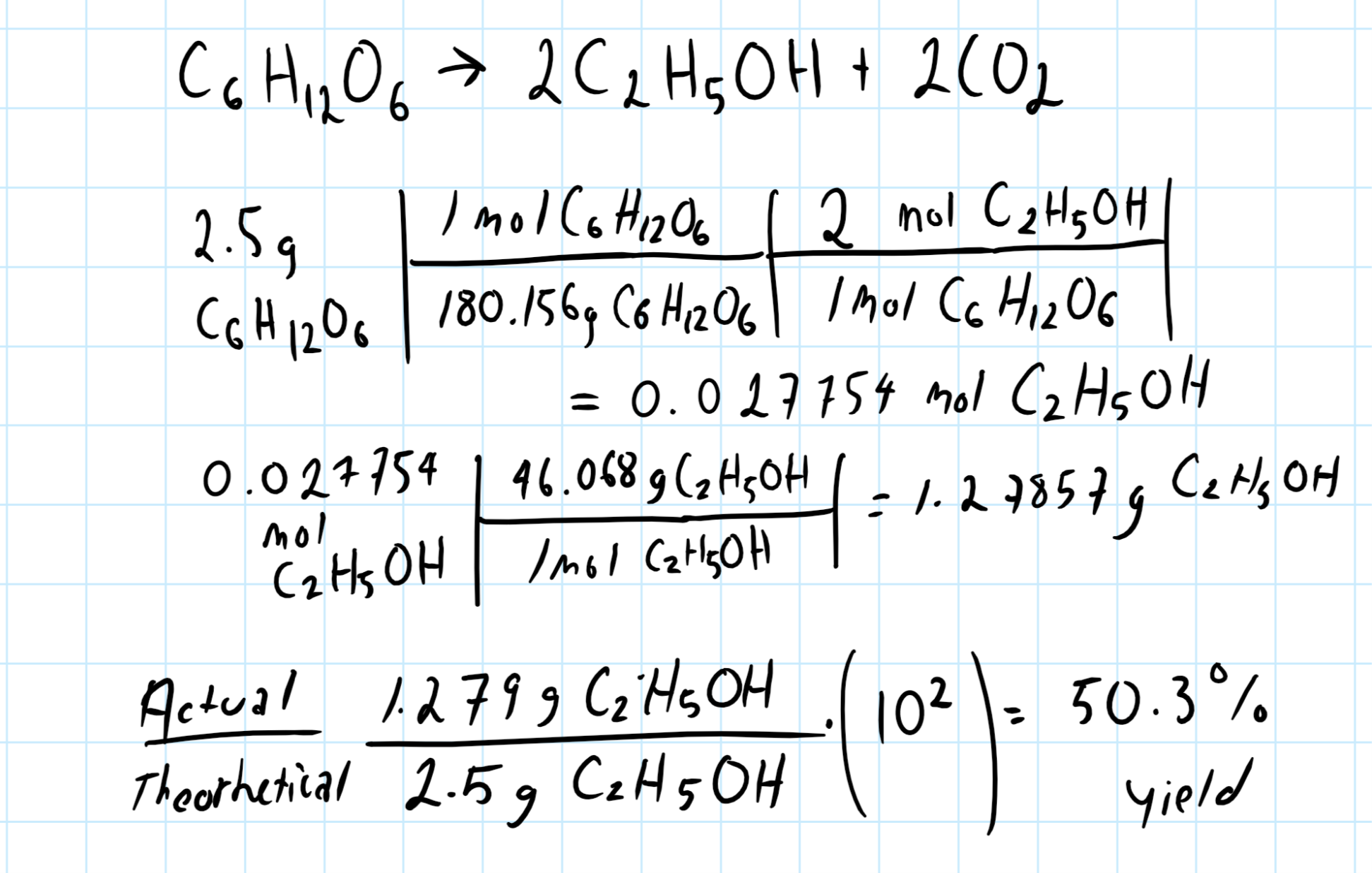Saccharomyces cerevisiae produces ethanol by fermentation. The theoretical yield of ethanol from 2.5 g of glucose is ______________ g ?
1 Answer
Dec 5, 2016
50.3% yield
Explanation:
Let's start by finding the balanced chemical reaction:
Remember that the percent yield is the actual yield divided by the theoretical yield multiplied by 100.
See if you can follow the graphic below, with this information you have provided, this is all I can do: