Show all the steps to how 3-chloroaniline can synthesis from benzene ?
1 Answer
Aug 9, 2018
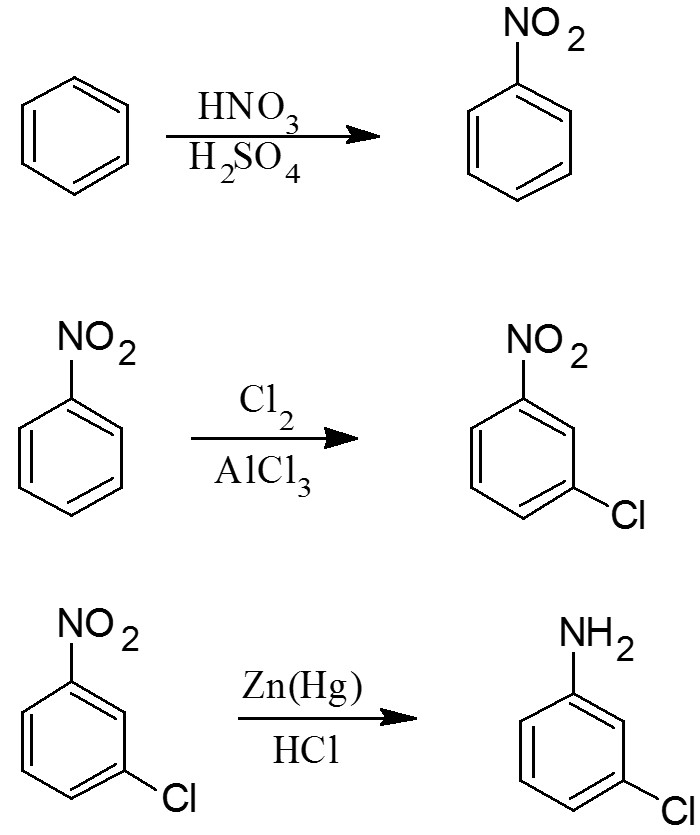
- nitration of benzene
- chlorination of nitrobenzene
- reduction of nitro group
Explanation:
Here's what the question is asking:
The synthesis will involve adding 2 substituents to the benzene ring. In this case, we'll need to consider the order of substitution.
- Cl is an ortho-para director . That means, if Cl is first added to benzene, the next substituent will be placed in the ortho/para position. That, will not work since, in the desired product,
#NH_2# is meta to Cl. - That means, we'll need to add Cl last.
#NH_2# is also an ortho-para director , so that means we can't add Cl after forming aniline.
An alternative we can consider is:
- First add nitro group (
#NO_2# ) to the benzene ring to form nitrobenzene through nitration of benzene. - Since nitro group is a meta director , we can then add Cl through chlorination .
- After that, we can reduce the
#NO_2# group to#NH_2# group.
Here's a possible synthesis you can consider: