Sodium hydroxide is added to a solution with a pH of two and thymol blue indicator. Describe the color of the solution at the following pH values: 4, 7, 10, and 13?
1 Answer
Feb 25, 2018
Here's what I get.
Explanation:
Thymol blue changes colour at two different pH ranges.
The first colour change is from red to yellow at pH 1.2–2.8, and the second is from yellow to blue at pH 8.0 - 9.6.
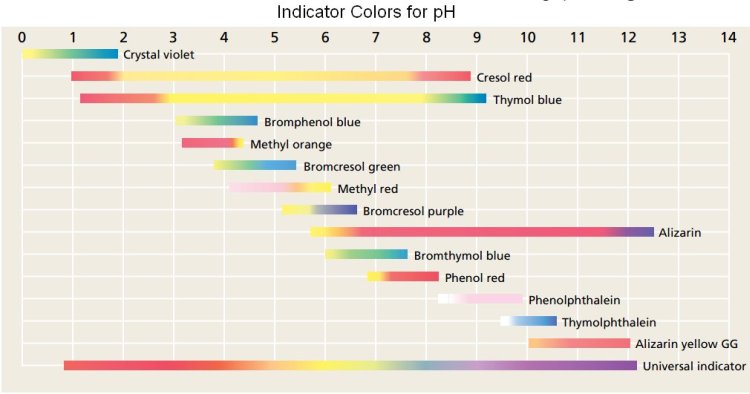
At pH 2, the indicator is in the middle of its first colour change, so the solution will be
At pH 4, the indicator is
At pH 7, the indicator is
At pH 10, the indicator is past its second colour change, so the solution will be
At pH 13, the solution will still be

