Solutions of the #[V(OH_2)_6]^(3+)# ion are green and absorb light of wavelength 560 nm. What is the ligand field splitting in the complex in kJ/mol?
1 Answer
I got
Well, the HOMO-LUMO gap in transition metal complexes tends to correspond to the
Vanadium as a
But that IS the octahedral ligand-field splitting energy,
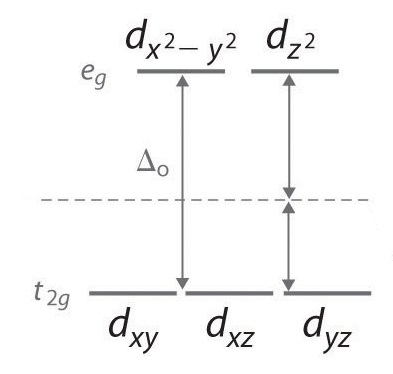
So, all you need to do is convert the wavelength to an energy. Recall:
#DeltaE = hnu = (hc)/lambda#
Therefore, we analogously have:
#Delta_o = (hc)/(lambda)#
#= ((6.626xx10^(-34) "J"cdotcancel"s")(2.998xx10^8 cancel"m""/"cancel"s"))/(560 cancel"nm" xx (cancel"1 m")/(10^9 cancel"nm"))#
#= 3.5473 xx 10^(-19) "J"#
Therefore, we need to convert to
#3.5473 xx 10^(-19) cancel"J" xx "1 kJ"/(1000 cancel"J") xx (6.0221413xx10^(23))/("1 mol complex")#
#=> color(blue)(Delta_o) =# #color(blue)("213.62 kJ/mol")#

