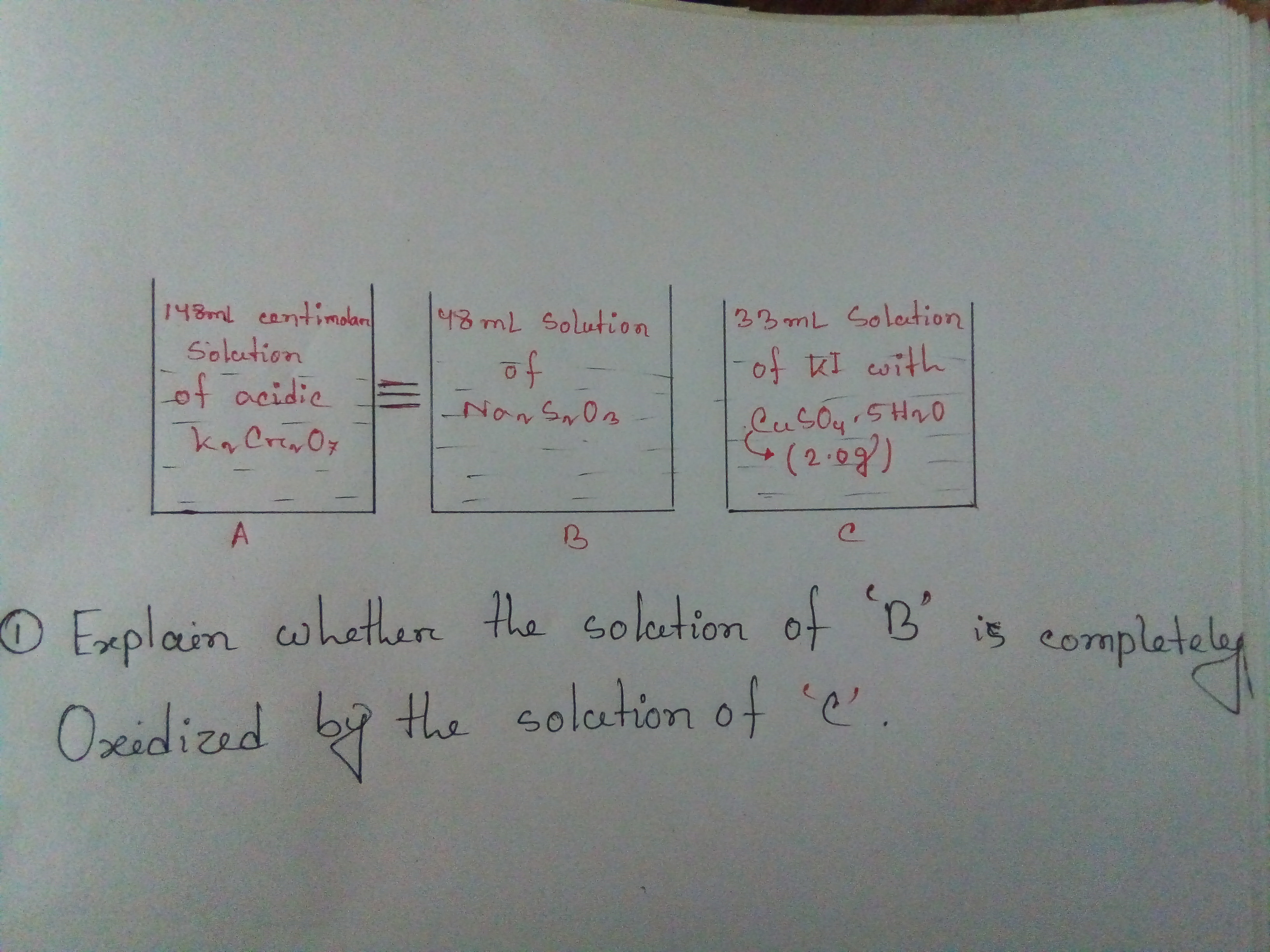
Step 1. Calculate the moles of "Cr"_2"O"_7^"2-" in Beaker A
"Moles of Cr"_2"O"_7^"2-" = 0.148 color(red)(cancel(color(black)("L Cr"_2"O"_7^"2-"))) × ("0.01 mol Cr"_2"O"_7^"2-")/(1 color(red)(cancel(color(black)("L Cr"_2"O"_7^"2-")))) = "0.001 48 mol Cr"_2"O"_7^"2-"
Step 2. Calculate the moles of "S"_2"O"_3^"2-" in Beaker B
The stoichiometric equation for a reaction between dichromate and thiosulfate is
"Cr"_2"O"_7^"2-" + 6"S"_2"O"_3^"2-" + 14"H"^"+" → "2Cr"^"3+" +"3S"_4"O"_6^"2-" +7"H"_2"O"
"Moles of S"_2"O"_3^"2-" = "0.001 48" color(red)(cancel(color(black)("mol Cr"_2"O"_7^"2-"))) × ("6 mol S"_2"O"_3^"2-")/(1 color(red)(cancel(color(black)("mol Cr"_2"O"_7^"2-")))) = "0.008 88 mol S"_2"O"_3^"2-"
3. Calculate the moles of "I"_2 formed in Beaker C
The "I"^"-" in Beaker C will react with the "Cu"^"2+" to form "I"_2.
"2Cu"^"2+" + "2I"^"-" → "2Cu"^"+" + "I"_2
If the "KI" is in excess, the "CuSO"_4 will be the limiting reactant.
"Moles of Cu"^"2+"
= 2.0 color(red)(cancel(color(black)("g CuSO"_4·5"H"_2"O"))) × (1 color(red)(cancel(color(black)("mol CuSO"_4·5"H"_2"O"))))/( 249.68 color(red)(cancel(color(black)("g CuSO"_4·5"H"_2"O")))) × "1 mol Cu"^"2+"/(1 color(red)(cancel(color(black)("mol CuSO"_4·5"H"_2"O")))) = "0.008 01 mol Cu"^"2+"
"Moles of I"_2color(white)(l) "formed" = "0.008 01" color(red)(cancel(color(black)("mol Cu"^"2+"))) × "1 mol I"_2/(2 color(red)(cancel(color(black)("mol Cu"^"2+")))) = "0.004 00 mol I"_2
Step 4. Calculate the moles of iodine needed to react with Beaker B
The equation for the reaction is
2"S"_2"O"_3^"2-" + "I"_2 → "2I"^"-" + "S"_4"O"_6^"2-"
"Moles of I"_2 = "0.008 88" color(red)(cancel(color(black)("mol S"_2"O"_3^"2-"))) × "1 mol I"_2/(2 color(red)(cancel(color(black)("mol S"_2"O"_3^"2-"))))= "0.004 44 mol I"_2
Step 5. Was there enough iodine in Beaker C to completely oxidize the contents of beaker C?
Beaker C contained 0.004 00 mol "I"_2.
Beaker B required 0.004 44 mol "I"_2.
Thus Beaker C did not contain enough iodine to completely oxidize the contents of Beaker B
The contents of Beaker B were not completely oxidized by the contents of Beaker C.