The activation energy of the reaction, A+B----> C+D+ 38kcal is 20kcal. What would be the activation energy of the reaction, C+D -----> A+B?
1 Answer
Mar 13, 2018
I'm going to say it is about 58kcal.
Explanation:
We need an exothermic plot. Here is a simple one.
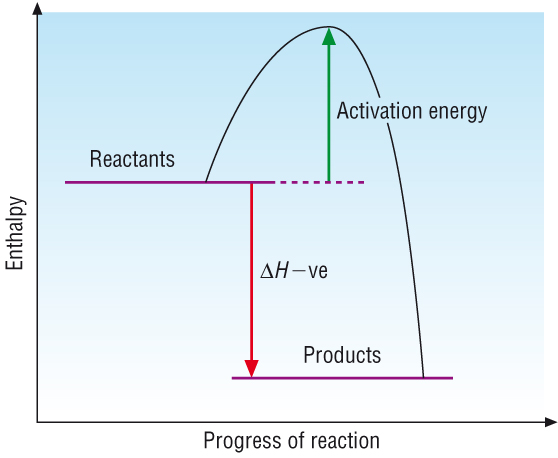
Ok, so for the process you are talking about above, the green arrow is 20kcal, and the red arrow is 38kcal.
The forward reaction is:
A+B -----> C+D
C+D -----> A+B means "go backwards", or in the reverse. So if you are down at products, you have to go all the way up to the top of the peak (this is your energy of activation...to take your reactants and get them to the activated state), then that distance (in energy) is 58kcal.
