The concentration of carbon monoxide in an urban apartment is #48# #µg##/##m^3#. What mass of carbon monoxide in grams is present in a room measuring 11.0 ft by 11.5 ft by 20.5 ft?
1 Answer
Explanation:
The idea here is that you need to use the information provided by the problem to find the volume of the room.
Since you are told that the concentration of carbon monoxide in a typical apartment is equal to
Now, you can treat the room as a rectangular prism of length
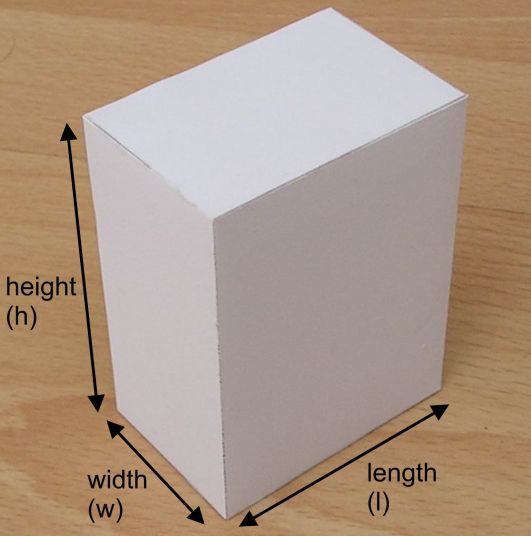
The volume of a rectangular prism is given by the formula
#color(blue)(V = l xx w xx h)#
You can use the conversion factor that exists between feet and meters to find the dimensions of the room in meters, then calculate the volume in cubic meters.
#"1 ft " = " 0.3048 m"#
The volume of the room will thus be
#V = (20.5color(red)(cancel(color(black)("ft"))) * "0.3048 m"/(1color(red)(cancel(color(black)("ft"))))) xx (11.5color(red)(cancel(color(black)("ft"))) * "0.3048 m"/(1color(red)(cancel(color(black)("ft"))))) xx (11.0color(red)(cancel(color(black)("ft"))) * "0.3048 m"/(1color(red)(cancel(color(black)("ft")))))#
#V = "73.43 m"^3#
This means that the room will contain
#73.43color(red)(cancel(color(black)("m"^3))) * overbrace( (48color(white)(a)mu"g")/(1color(red)(cancel(color(black)("m"^3)))))^(color(purple)("given concentration")) = 3524.64mu"g"#
Rounded to two sig figs, the number of sig figs you have for the concentration of carbon monoxide, the answer will be
#M_(CO) = color(green)(3500color(white)(a)mu"g")#

