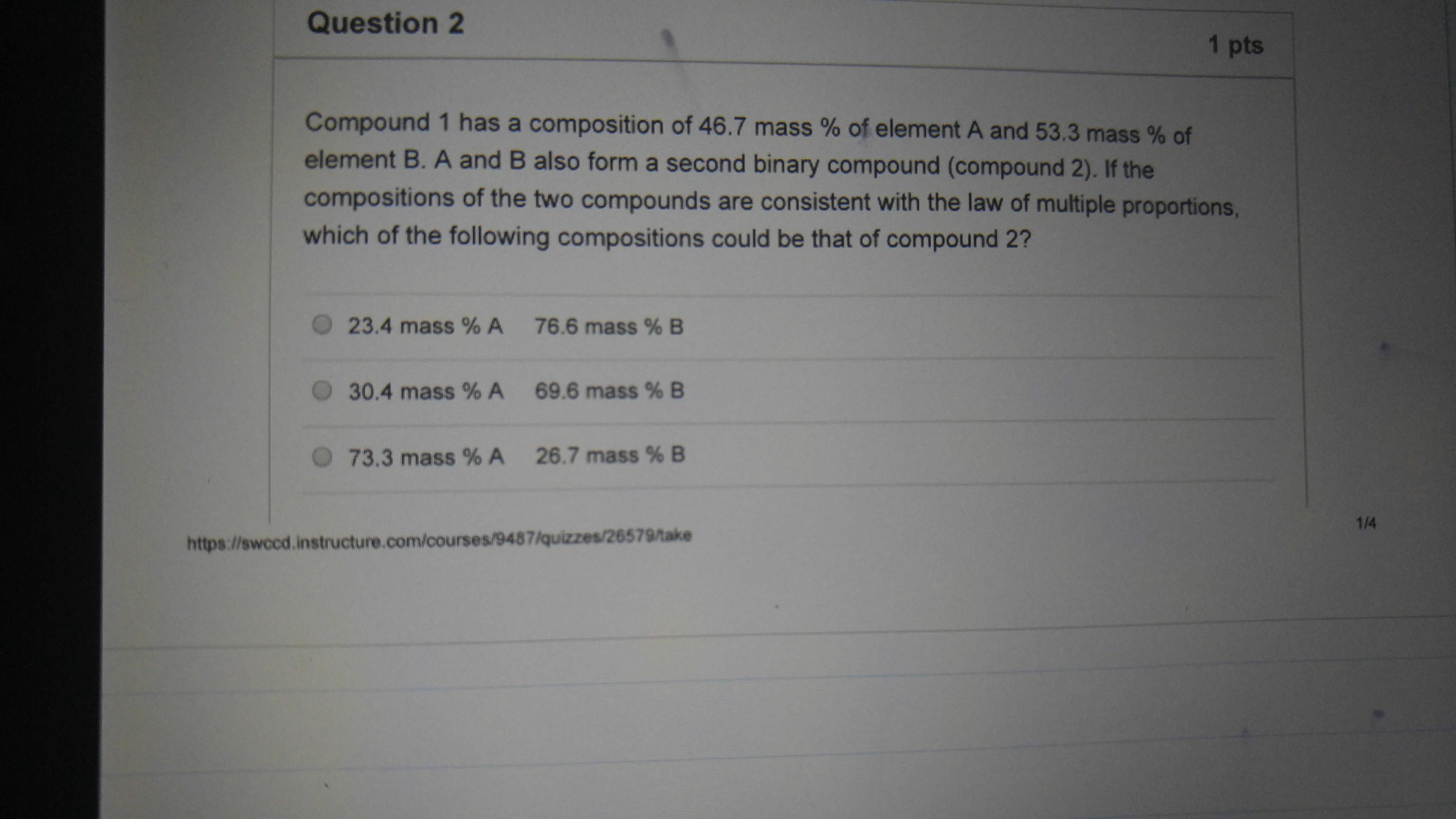
This question is using the concept Law of multiple proportions, how cn I use that law to solve this problem?
1 Answer
Option 2 has the correct ratio for Compound 2.
Explanation:
The Law of Multiple Proportions states that when two elements A and B combine to form two or more compounds, the masses of B that combine with a given mass of A are in the ratios of small whole numbers.
Compound 1
In Compound 1, the mass ratio of A:B is
If we divide both sides of the ratio by 46.7, it becomes
In other words, 1 g A combines with 1.14 g B.
Law of Multiple Proportions
Now, let's do the same calculation for each of the options given for Compound 2.
In Compound 1, 1 g A reacts with 1.14 g B
In Compound 2, 1 g A reacts with 2.28 g B
The ratio of the masses of B in the two compounds is
Option 2 has the correct ratio for Compound 2.

