Titration curve? Please help...
50ml of 0.100M H3PO4 is titrated with 0.100M NaOH (i) calculate the pH after the addtion of the following volumes of titrant; 0.00, 10.00, 25.00, 50.00, 65.00, 75.00, 100.00, and 110.00. Note that since Ka1 is relatively large, the complete quadratic should be solved for the first three volumes. (ii) Plot the titration curve.
answers (i) 1.62; 1.89; 2.26; 4.67; 6.84; 7.21; 9.77; 11.72; (ii) Graph
50ml of 0.100M H3PO4 is titrated with 0.100M NaOH (i) calculate the pH after the addtion of the following volumes of titrant; 0.00, 10.00, 25.00, 50.00, 65.00, 75.00, 100.00, and 110.00. Note that since Ka1 is relatively large, the complete quadratic should be solved for the first three volumes. (ii) Plot the titration curve.
answers (i) 1.62; 1.89; 2.26; 4.67; 6.84; 7.21; 9.77; 11.72; (ii) Graph
1 Answer
Well, first I would sketch the titration curve of a diprotic acid (
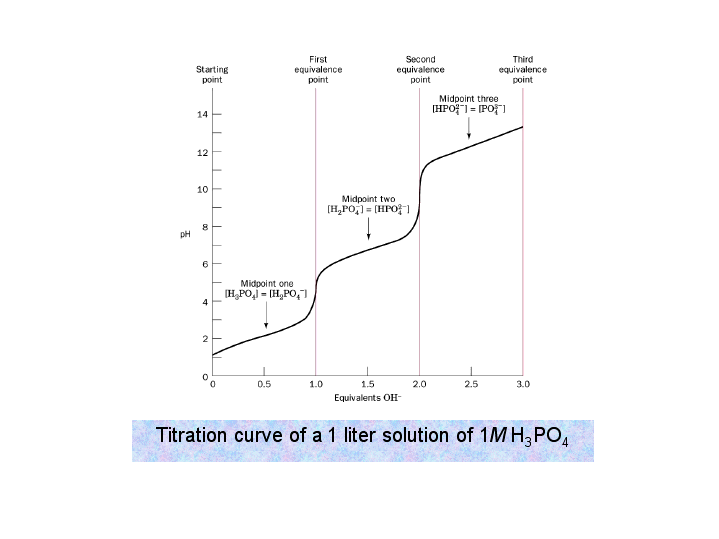
Here we distinguish between the buffer region (wherein the half-equivalence point lies) and the equivalence point.
- Buffer region --- Henderson-Hasselbalch equation does NOT apply UNLESS the current
#K_a# is small.#K_(a1)# is not small enough, but#K_(a2)# and#K_(a3)# are. - Half-equivalence point ---
#"pH" = "pK"_a# - Equivalence point --- Regular weak acid/base equilibrium!
#K_(a1,2,3) = 7.1 xx 10^(-3), 6.3 xx 10^(-8), 4.5 xx 10^(-13)#
So we first find the equivalence points.
#"H"_3"PO"_4 + 3"NaOH"(aq) -> "Na"_3"PO"_4(aq) + 3"H"_2"O"(l)#
#"0.100 mol"/"L" xx "0.050 L" = "0.0050 mols H"_3"PO"_4#
#= "0.0150 mols OH"^(-)#
#=> "1000 mL"/"0.100 mol NaOH" xx "0.0150 mols OH"^(-)#
#=# #"150 mL"# for all three protons
#=># #"50 mL"# for each proton
Thus, the equivalence points are at
#"H"_3"PO"_4(aq) rightleftharpoons "H"_2"PO"_4^(-)(aq) + "H"^(+)(aq)#
#K_(a1) = 7.1 xx 10^(-3) = (["H"_2"PO"_4^(-)]["H"^(+)])/(["H"_3"PO"_4]) = x^2/(0.100 - x)# Although
#x# is not small enough, since#(K_(a1))/("0.100 M") < 1# , this can be done iteratively.
#x_1 ~~ sqrt(0.100K_(a1)) = "0.02665 M"#
#x_2 ~~ sqrt((0.100 - x_1)K_(a1)) = "0.02282 M"#
#x_3 ~~ sqrt((0.100 - x_2)K_(a1)) = "0.02341 M"#
#x_4 ~~ sqrt((0.100 - x_3)K_(a1)) = "0.02332 M"#
#x_5 = sqrt((0.100 - x_4)K_(a1)) = "0.02333 M"# And thus,
#x = "0.02333 M"# for#["H"^(+)]# . That gives
#color(blue)("pH"_1) = -log(0.02333) = color(blue)(1.63)# .
#"0.100 mol/L" xx "0.010 L" = "0.0010 mols OH"^(-)# This neutralizes that much
#"H"_3"PO"_4# to give
#"0.0050 mols H"_3"PO"_4 - "0.0010 mols OH"^(-)#
#= "0.0040 mols H"_3"PO"_4# leftover
#-> "0.0010 mols H"_2"PO"_4^(-)# producedThis is then a concentration of
#("0.0010 mols H"_2"PO"_4^(-))/("50.00 mL acid" + "10.00 mL base") cdot "1000 mL"/"1 L"#
#=# #"0.01667 M H"_2"PO"_4^(-)# and
#"0.06667 M H"_3"PO"_4# These again go into the full equilibrium expression. Remember to include the initial concentrations.
#K_(a1) = 7.1 xx 10^(-3) = (["H"_2"PO"_4^(-)]["H"^(+)])/(["H"_3"PO"_4])#
#= ((0.01667 + x)(x))/(0.06667 - x)# This must be solved in full.
#7.1 xx 10^(-3) cdot 0.06667 - 7.1 xx 10^(-3)x = 0.01667x + x^2#
#x^2 + (0.01667 + 7.1 xx 10^(-3))x - 7.1 xx 10^(-3) cdot 0.06667 = 0#
#x^2 + 0.02377x - 4.73 xx 10^(-4) = 0# and here we get
#["H"^(+)] = x = "0.01290 M"# , so
#color(blue)("pH"_2) = -log(0.01290) = color(blue)(1.89)#
I won't show much math here, but you can convince yourself that you are at the first half-equivalence point, so
#"pH"_3 = "pK"_(a1) = -log(7.1 xx 10^(-3)) ~~ 2.15# is expected.However, it's a bit larger, due to Le Chatelier's principle.
#["H"_2"PO"_4^(-)] = ["H"_3"PO"_4] = "0.0025 mols"/("50.00 mL acid" + "25.00 mL base") cdot "1000 mL"/"1 L"#
#=# #"0.03333 M"#
#K_(a1) = 7.1 xx 10^(-3) = (["H"_2"PO"_4^(-)]["H"^(+)])/(["H"_3"PO"_4])#
#= ((0.03333 + x)(x))/(0.03333 - x)# Solving this, we find
#0 = x^2 + (0.03333 + 7.1 xx 10^(-3))x - 7.1 xx 10^(-3) cdot 0.03333# for which
#x = ["H"^(+)] = "0.005188 M"# and
#color(blue)("pH"_3) = -log(0.005188) = color(blue)(2.29)#
Here we are halfway between the first and second half-equivalence points, which had
#"pH" = "pK"_(ai)# , so
#color(blue)("pH"_4 = 1/2("pK"_(a1) + "pK"_(a2)) = 4.67)#
#"0.0015 mols NaOH"# added to neutralize#"0.0015 mols H"_2"PO"_4^(-)# to give#"0.0015 mols HPO"_4^(2-)# and#"0.0035 mols H"_2"PO"_4^(-)# in#"50.00 + 65.00 mL"# solution.The Henderson-Hasselbalch equation here would apply, since
#K_(a2)# is small. The#K_(a2)# would be:
#6.3 xx 10^(-8) = ((0.01304 + x)(x))/(0.03043 - x) ~~ (0.01304x)/0.03043#
#=> x ~~ 1.470 xx 10^(-7) "M"# and
#color(blue)("pH"_5 ~~ 6.83)# .Or, we could have done...
#"pH"_5 = "pK"_(a2) + log((["HPO"_4^(2-)])/(["H"_2"PO"_4^(-)]))#
#= -log(6.3 xx 10^(-8)) + log(0.01304/0.03043)#
#= 6.83#
And so, we get
#["H"_2"PO"_4^(-)] = ["HPO"_4^(2-)]# , which means
#color(blue)("pH"_6) ~~ "pK"_(a2) = -log(6.3 xx 10^(-8))#
#= color(blue)(7.20)#
Here we are halfway between the second and third half-equivalence points, which had
#"pH" = "pK"_(ai)# , so
#color(blue)("pH"_7 = 1/2("pK"_(a2) + "pK"_(a3)) = 9.77)#
Therefore, we are
#"0.100 mol/L" xx "0.010 L" = "0.0010 mols OH"^(-)# past the second equivalence point, which means we neutralized
#"0.0010 mols HPO"_4^(2-)# and made#"0.0010 mols PO"_4^(3-)# . This gives
#["HPO"_4^(2-)] = ("0.0040 mols HPO"_4^(2-))/("50.00 + 110.00 mL") cdot "1000 mL"/"1 L"#
#=# #"0.02500 M"#
#["PO"_4^(3-)] = ("0.0010 mols HPO"_4^(2-))/("50.00 + 110.00 mL") cdot "1000 mL"/"1 L"#
#=# #"0.00625 M"# As a result, and we can again make small
#x# approximations here,
#K_(a3) = 4.5 xx 10^(-13) = ((0.00625 + x)(x))/(0.02500 - x)#
#~~ 0.00625/0.02500 x#
#=> x ~~ 1.80 xx 10^(-12) "M"# And that gives
#color(blue)("pH"_8) = -log(1.80 xx 10^(-12)) = color(blue)(11.74)#
Here we "derive"
#"pH"_("1st-half-equiv") = "pK"_(a1) + log((["base"])/(["acid"]))#
#"pH"_("2nd-half-equiv") = "pK"_(a2) + log((["base"])/(["acid"]))#
At the half-equivalence point, the concentrations of weak base and weak acid are equal, so
A perfect titration would give a
That is,
-
the
#"pH"# on either side of the equivalence point is equidistant from the equivalence point#"pH"# . -
the
#"pH"# on either side of the half-equivalence point is equidistant from the half-equivalence point#"pH"# .
Therefore, if we want the second equivalence point, we are equidistant from the second half-equivalence point and third half-equivalence point, i.e.
#"pH"_("equivpt",i) = 1/2("pK"_(ai) + "pK"_(a(i+1)))#
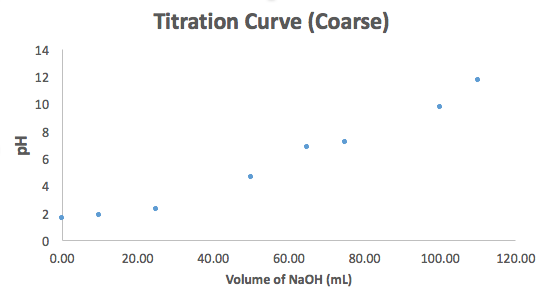
Can you label this graph with the half-equivalence and equivalence points? (There are two of each on this graph.)

