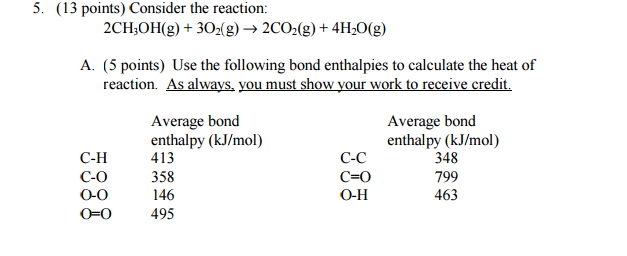
Use the following bond enthalpies to calculate the heat of reaction?
1 Answer
For
#2"CH"_3"OH"(g) + 3"O"_2(g) -> 2"CO"_2(g) + 4"H"_2"O"(g),#
we have to determine which bonds have been broken and made. It really helps to draw the Lewis structures. Good thing your reaction is balanced.
We assume that the stoichiometric coefficients have scaled the reaction to a per-mol basis. For instance, we assume that there are
LEWIS STRUCTURE REPRESENTATIONS
Oxygen reacts in a combustion reaction as
BOND-BREAKING/FORMING ACCOUNT
Reactants side
- 6
#"mol"# s of#"C"-"H"# sigma bonds broken - 2
#"mol"# s of#"C"-"O"# sigma bonds broken - 2
#"mol"# s of#"O"-"H"# sigma bonds broken
- 3
#"mol"# s of#"O"="O"# double bonds broken
Products side
- 4
#"mol"# s of#"C"="O"# [sigma+pi] bonds formed
- 8
#"mol"# s of#"O"-"H"# sigma bonds formed
SIGN CONVENTIONS
-
Bonds broken
#-># #\mathbf(Delta"H"_"bond" > 0)# because potential energy is stored into the bond to break the bond. -
Bonds formed
#-># #\mathbf(Delta"H"_"bond" < 0)# because potential energy is released from the bond upon forming the bond.
RESULTS
You weren't told what mass of methanol you started with, so I'm assuming you're supposed to calculate the ENTHALPY of reaction, NOT the heat flow of the reaction (these are NOT the same!).
Thus, the approximate enthalpy of reaction, having already accounted for the stoichiometries, is:
#color(blue)(Delta"H"_"rxn")#
#~~ sum Delta"H"_"broken" - sum Delta"H"_"formed"#
#= [6Delta"H"_("C"-"H") + 2Delta"H"_("C"-"O") + 2Delta"H"_("O"-"H") + 3Delta"H"_("O"-"O")] - [4Delta"H"_("C"="O") + 8Delta"H"_("O"-"H")]#
#= [6(413) + 2(358) + 2(463) + 3(495)] - [4(799) + 8(463)]# #"kJ/mol"#
#= color(blue)(-"1295 kJ/mol")#
which makes sense to be negative, because it's a combustion reaction.

