Water and oil are placed in the same vessel and stirred. What is the reason that oil and water will not mix?
1 Answer
Apr 5, 2017
Water is highly polar and oil is nonpolar.
Explanation:
Nonpolar and polar molecules do not mix.
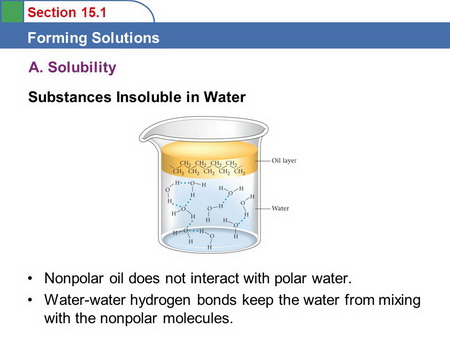
(From slideplayer.com)
The water molecules are attracted to each other by strong hydrogen bonds.
They have little incentive to move into the oil phase.
The oil molecules are attracted to each other by London dispersion forces.
Their attractive forces are so weak that they are unable to force the water molecules apart and move into the water phase.

