What are coulombic and exchange energy and how are they determined?
1 Answer
A simplified way is to call them
Pi = Pi_c + Pi_e
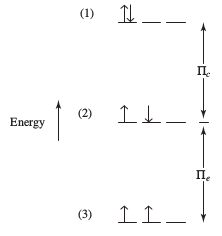
[This is gone into more detail here.]
For example, consider the ground state valence configuration of chromium:
ul(uarr color(white)(darr))
4s
underbrace(ul(uarr color(white)(darr))" "ul(uarr color(white)(darr))" "ul(uarr color(white)(darr))" "ul(uarr color(white)(darr))" "ul(uarr color(white)(darr)))
" "" "" "" "" "" "color(white)(/)3d
This has zero coulombic repulsion energy
Label each electron to get:
uarr_1 uarr_2 uarr_3 uarr _4 uarr_5
Now, the possible exchanges (that result in the same energy) involve all of the
1harr{2, 3, 4, 5} -> 4 exchanges
2harr{3, 4, 5} -> 3 exchanges
3harr{4, 5} -> 2 exchanges
4harr{5} -> 1 exchange(Try not to double-count; so don't count
1harr2 and then2harr1 ; those are the same thing.)
This is true because all of the electrons are all indistinguishable. This results in an exchange energy stabilization of
So the pairing energy in total is:
Pi = 10Pi_e
Now, suppose instead, the chromium atom was in this state:
ul(uarr darr)
4s
underbrace(ul(uarr color(white)(darr))" "ul(uarr color(white)(darr))" "ul(uarr color(white)(darr))" "ul(uarr color(white)(darr))" "ul(color(white)(uarr darr)))
" "" "" "" "" "" "color(white)(/)3d
Now there is one pair of electrons that ARE repelling each other, so the coulombic repulsion energy is
So the pairing energy in total is higher than before:
Pi = Pi_c + 6Pi_e
This is less stable by
This approach also explains why tungsten prefers a

