What are products of these Hoffman eliminations?
1 Answer
Here's what I think.
Explanation:
The Hofmann elimination is a process in which an amine is converted to a quaternary ammonium salt by treatment with excess methyl iodide and then reacted with silver oxide and water to form an alkene.
The silver oxide and water form hydroxide ions which eliminate a β-hydrogen.
For example,
The major product is the least substituted alkene.
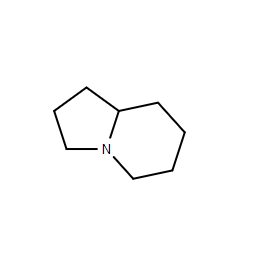
Hofmann elimination of 1-azabicyclo[4.3.0]nonane
I would expect the quaternary ammonium salt to give a mixture of two alkenes.
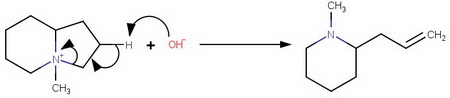
One is N-methyl-2-(prop-2-en-1-yl)piperidine, formed by elimination from
the 5-membered ring.
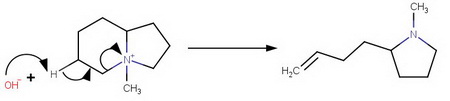
The other is 2-(but-3-en-1-yl)-N-methylpyrollidine, formed by elimination from
the 6-membered ring.
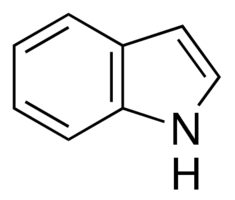
I would not expect indole to undergo Hofmann elimination because it has no aliphatic β-hydrogens to be eliminated.

