What are strong acids or bases are that ionize or dissociate in aqueous solution?
1 Answer
Strong acids and bases are those which have the tendency to easily dissociate into ions in aqueous solution.
Explanation:
Strong acids and strong bases are those species which dissociate/ionize essentially to completion upon being placed in aqueous solution.
Dissociation of a strong acid (hydrochloric for example):
#"HCl"(g) rarr "H"^+(aq) + "Cl"^(-)(aq)# Dissociation of a strong base (sodium hydroxide for example):
#"NaOH"(s) rarr "Na"^+ (aq) + "OH"^(-) (aq)#
Substances that ionize completely (which include strong acids and bases) are called strong electrolytes, and further include basically all water-soluble ionic compounds.
For a list of the strong acids and bases, try this:
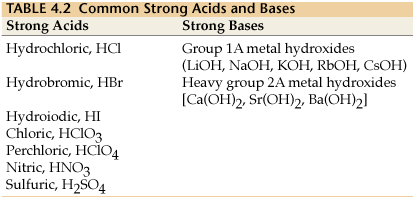
For sulfuric acid,

