What are the brackets for around the Lewis structure?
1 Answer
Jan 29, 2017
They allow one to unambiguously describe the charge of the overall ion. Here is an example:
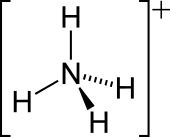
For
(If you recall, formal charge assumes evenly-shared valence electrons and is

