What are the characteristics/properties of the noble gases?
1 Answer
Jul 22, 2018
Well, the Noble Gases are all colourless, room temperature gases.
Explanation:
….and by virtue of their closed shell, electronic configuration, they are (i) difficult to oxidize, and (ii) difficult to reduce.. Given this lack of reactivity, their chemistry is quite limited, and they tend to only react with the MOST reactive elements, i.e. difluorine, and dioxygen.
As the elements, these are all room temperature gases, with only dispersion forces operating as the intermolecular (interatomic force).
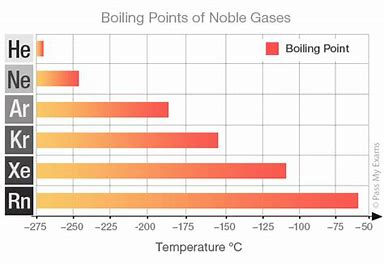
As is typical, the more electrons the gas has, the more intermolecular force, and thus the higher the boiling point.

