What are the factors on which ionization energy depends?
1 Answer
We assess the energy change for the process:
Explanation:
Two factors are important here: (i) nuclear charge; and (ii) shielding by other electrons. Incomplete electronic shells shield nuclear charge very ineffectively, with the result that ionization energies follow a strongly Periodic trend: the energies INCREASE across a Period from left to right as we face the Table, and DECREASE down a Group.
Using the usual electronic structure that helps us develop the Periodic Table, we can account for this. Incomplete, i.e. unfilled, electronic shells shield nuclear charge very ineffectively. Elements to the right of the Period (as we face it) should have higher ionization enthalpies, as the atomic number,
On the other hand, once a shell is filled, the valence electrons in the new shell should be easier to ionize. This effect is also observed in the well known decrease in ATOMIC radius across the Period from left to right. Why so?
As chemists, as physical scientists, however, our argument should be informed by data.
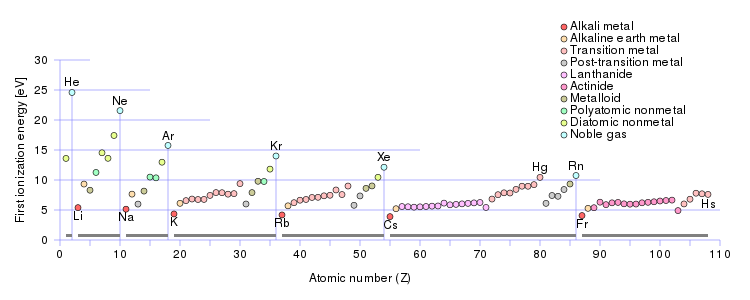
Here first ionization energy (in

