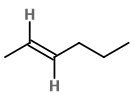
What are the geometrical isomers of 2-hexene?
2 Answers
Apr 27, 2018
Well you got an internal olefin....
Explanation:
...i.e.
Apr 27, 2018
The isomers are cis-hex-2-ene and trans-hex-2-ene.
Explanation:
cis-hex-2-ene
The cis isomer has the two
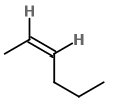
trans-hex-2-ene
The trans isomer has the two