What are the names, charges, and locations of the three types of subatomic particles that make up an atom?
1 Answer
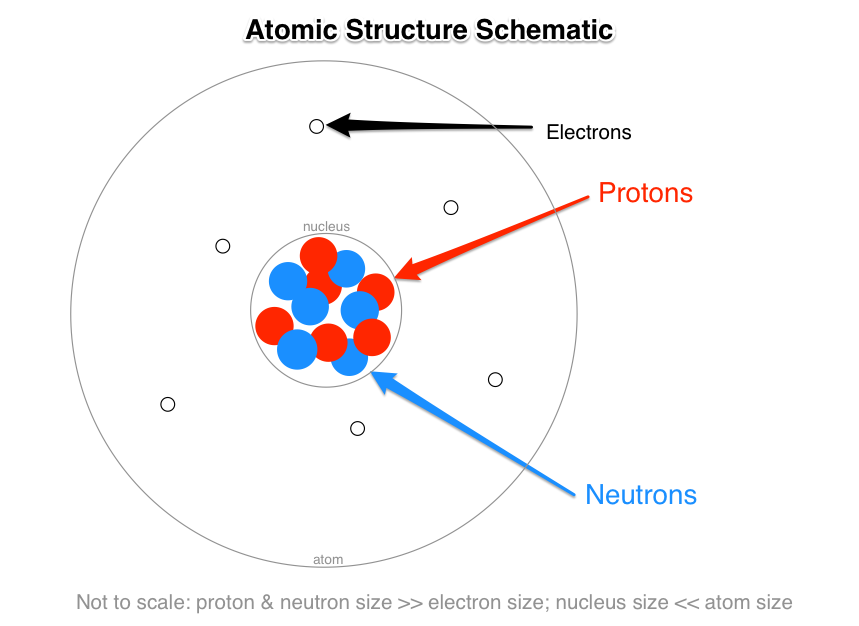
Proton (charge of +e, in the nucleus), Neutron (0 charge, in the nucleus), and Electron (charge of –e, outside the nucleus).
Explanation:
-
Proton. This is a positively charged particle that is present in the nucleus of atoms. It has a charge of
#+ 1.6 × 10^(-19) C# . But for ease we might say it has a charge of +e or +1. -
Neutron. This particle has a charge of zero; it is uncharged/neutral. It is present in the nucleus of atoms.
-
Electron. This is a negatively charged particle that orbits the nucleus of atoms, i.e. outside the nucleus (electrons exist in the space between atomic nuclei). They have a charge of
#– 1.6 × 10^(-19) C# . But for ease we might say it has a charge of –e or –1.
Charges
Charge is measure in coulombs (symbol, C). The elementary charge is

