What is the number of each subatomic particle in copper?
1 Answer
There are 29 protons, 35 neutrons, and 29 electrons in a copper atom.
Explanation:
You can find these numbers yourself by looking at a periodic table of the elements:
Copper will be the 29th element in the table reading from left to right, top to bottom. The box for copper will look something like this:
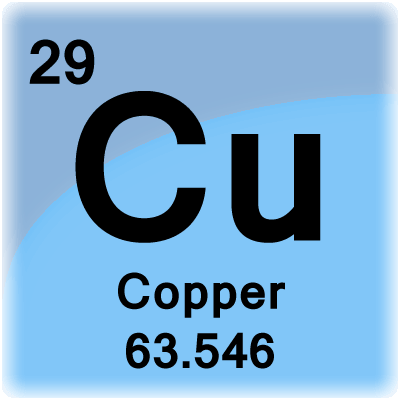
The number in the top-left corner is called the atomic number, and that is how many protons and electrons are in a normal copper atom.
The number at the bottom the atomic mass. If you round that number and subtract it from the atomic number then you will be left with the number of protons. Like this:
number of neutrons = atomic mass - atomic number
(In copper's case the most common isotope is Cu-63 so the number of neutrons would really be 34, but for middle/high school chemistry the teacher will just expect you to round the atomic mass.)

