What are the three possible Lewis structures for C2H2Cl2?
1 Answer
May 14, 2018
Here's what I get.
Explanation:
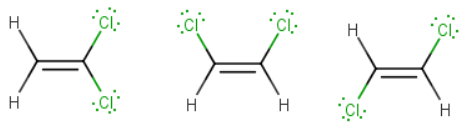 Image
Image
The first isomer has two chlorine atoms on the same carbon,
The π-bond in alkenes prevents rotation about the
Thus, thee are two isomers with the chlorine atoms on different carbons:
- one has the two chlorine atoms on the same side of the double bond
- the other has the two chlorine atoms on opposite sides of the double bond

