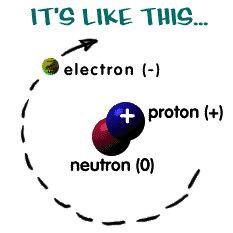
What are the three subatomic particles found inside an atom?
1 Answer
Aug 23, 2017
There are basically three subatomic particles inside an atom
Subatomic particles are those which make up an atom.They are:
It is the neutral part of an atom
It means that it has no charge. It is neither negative nor positive
It is situated in the nucleus or the centre part of an atom
It is the positive part of an atom
It has a positive charge
It is also located in the nucleus of an atom attached to a neutron or another proton
It is the negative part of an atom
It has a negative charge
It revolves around the nucleus of an atom