What causes the emission of radiant energy that produces characteristics spectral lines?
1 Answer
Dec 27, 2015
From Bohr Model of the atom we see that when an electron jumps from an allowed orbit to another, radiant energy (photon) is emitted.
Explanation:
You can have an idea of the phenomenon observing the emission spectrum of hydrogen and the transitions responsible for it:
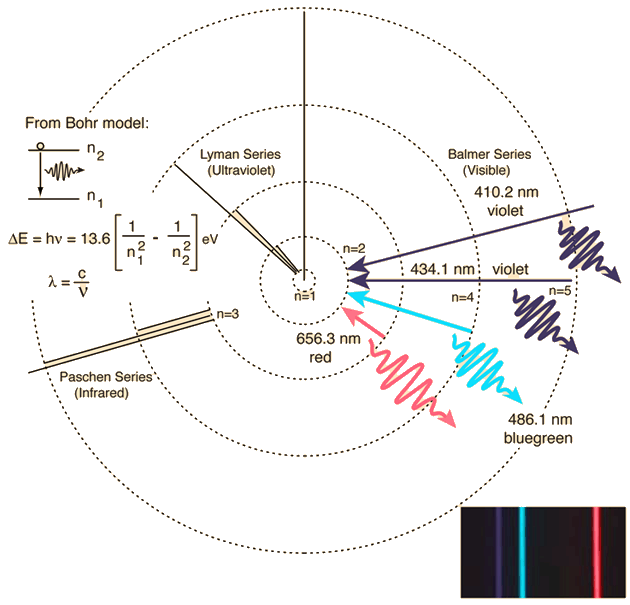
Red for example is emitted (and you see the red line in emission) when the electron "falls" from orbit at

