What do electrochemical cells include?
1 Answer
The basic components of an electrochemical cell will be two conductors to serve as electrodes plus an electrolytic solution in which the electrodes are immersed.
Explanation:
The electrodes are frequently two different metals. One serves to allow electrons to leave the cell at the location of the oxidation. This is the negative terminal, called the anode. The other electrode will bring the electrons back into the cell once their potential energy has been put to work. This is the positive terminal or cathode, and reduction will occur at this location.
The materials of which these terminals are made, may or may not be consumed as the chemical reaction takes place within the cell. If they are not consumed, they are referred to as inert electrodes.
A common choice for anode is zinc (in regular and alkaline dry cells), which is consumed in the reaction, while the cathode is a rod of carbon (graphite) and is not consumed. In the alkaline dry cell, the electrolyte is a moistens paste of KOH (a strong base).
In the storage battery used in vehicles, the electrodes are lead (anode) and a grid of lead (IV) oxide (cathode), with a concentrated solution of sulfuric acid serving as the electrolyte.
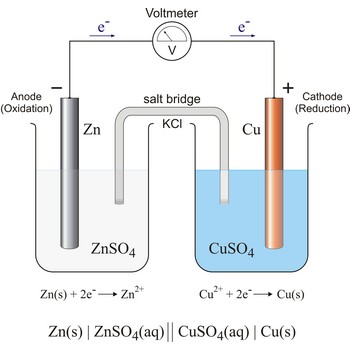
The diagram below shows the type of cell that most texts introduce as a first look at electrochemical cells. In it, the two electrodes are physically separated and a "salt bridge" is used to maintain electrical conductivity between them.