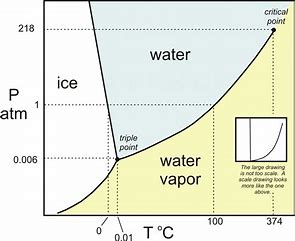
Consider the transition #"ice " rarr" water"#...now the slope #(DeltaP)/(DeltaT)# is CLEARLY negative....(mind you the graph exaggerates this slope). And thus #(DeltaP)/(DeltaT)="a negative slope"#...
And now we go to the Clapeyron equation for phase change...
#(DeltaP)/(DeltaT)-=(DeltaS)/(DeltaV)#...now given the stated transition, CLEARLY #DeltaS# is POSITIVE (the liquid state is more disordered than the solid state)....because we go from a SOLID phase, ice, to a liquid phase....and ACCORDINGLY, #DeltaV# MUST be negative to account for the slope. And thus the VOLUME of a given mass of ICE is GREATER than the volume of a given mass of WATER under equivalent conditions. The result is that ice floats....and this is an highly unusual condition for substances.


