What does binding energy measure?
1 Answer
Mar 7, 2016
Chemical bond energy is the amount of energy required to break a chemical bond starting with a molecule initially in its lowest-energy state and producing two fragments, each in its lowest-energy state.
Explanation:
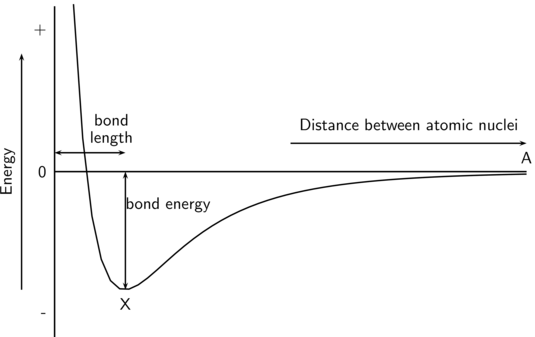
All chemical bonds have an equilibrium bond length which is the distance between nuclei corresponding to the lowest potential energy (point X in the figure below). The bond energy is the amount of energy required to separate the nuclei to an infinite distance (essentially, to point A in the diagram).