Electronegativity is the tendency of a bonded atom to attract electrons to itself. The difference in electronegativity (#Delta# EN) between bonded atoms can indicate whether the bond is nonpolar, polar covalent, or ionic. Generally, the farther apart two elements are on the periodic table, the more ionic the bond character, and the closer together they are, the less ionic the bond is.
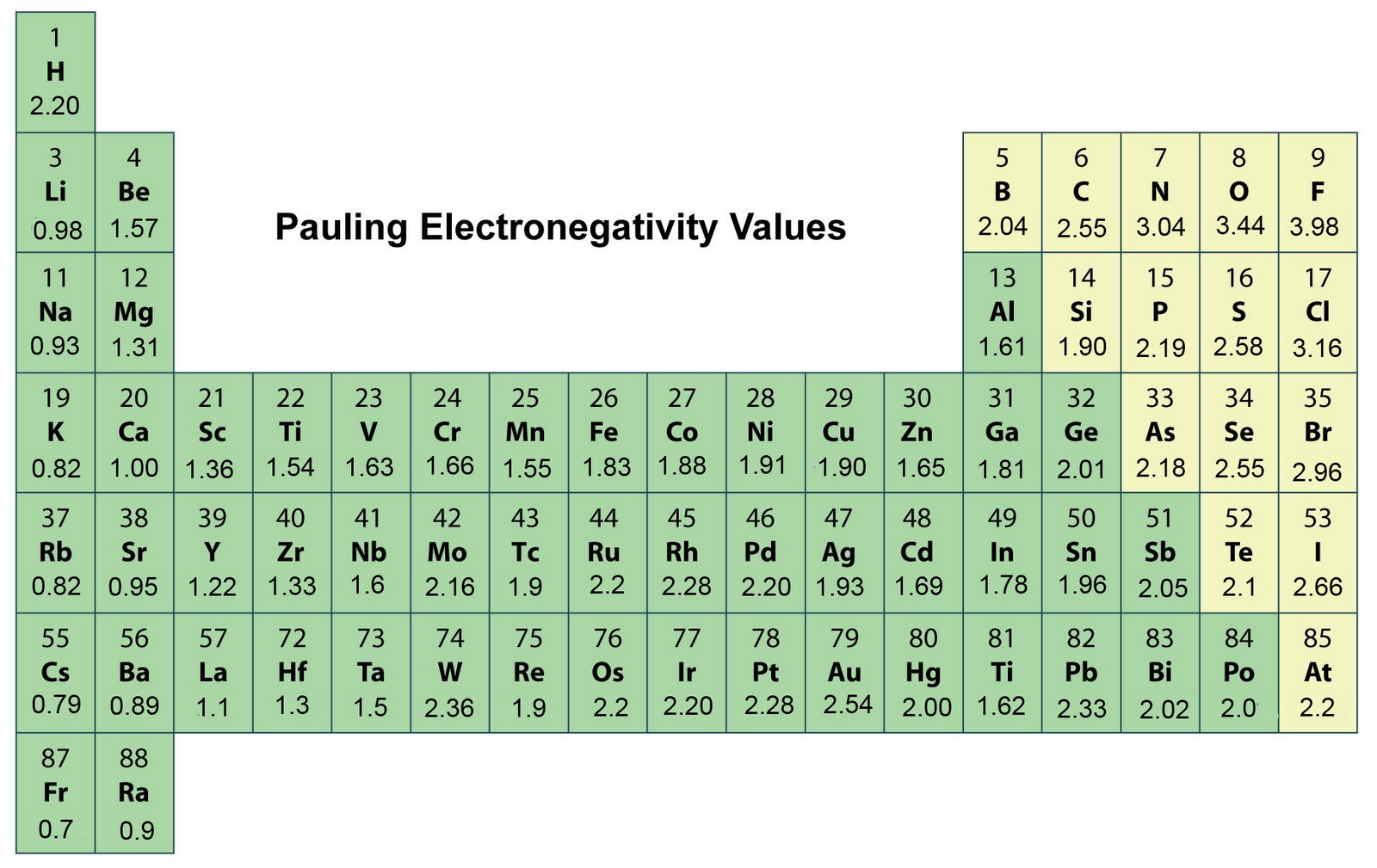
The table above has the electronegativities of the elements as determined by Linus Pauling.
1.#Delta# EN of less than 0.4 is considered to be nonpolar.
2.#Delta# EN of 0.5 to 1.7 are considered polar covalent.
3.#Delta# EN greater than 1.7 is considered ionic.
Different textbooks may have slightly different #Delta# EN values for determining the bond character.


