What does it mean if a molecule is polar?
1 Answer
When a molecule is polar, it means that it has positive and negative ends.
Explanation:
By definition, polarity is basically the status of having "poles."
In a molecule, this means that the molecule has poles of positive and negative charge—also known as a separation of charge.
Let's take a look at a classic example of polarity: the water molecule.
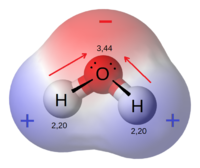
Oxygen is more electronegative than hydrogen.
This means that, in the
So, electrons in each
Therefore, these
In
- There is a center of negative charge at the top, where
#O# is. - There is a center of positive charge at the bottom, where
#H# is.
This existence of a positive and negative pole causes the entire

