What does the group number of a column in the periodic table represent?
1 Answer
Dec 2, 2015
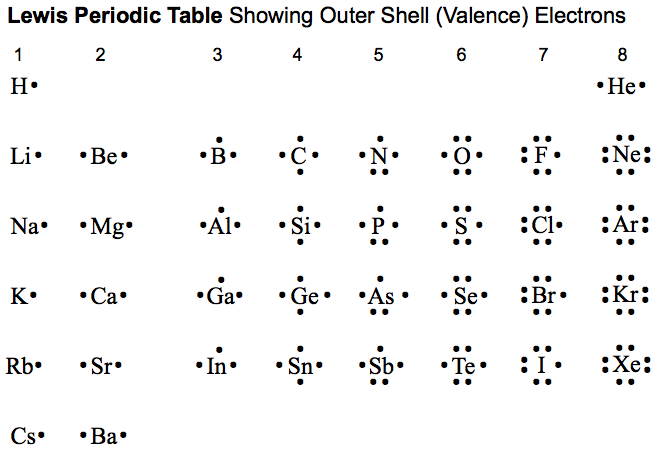
It represents the number of valence electrons for the elements in the group.
Explanation:
For the representative elements (main group elements), groups 1/IA-2/IIA and 13/IIIA to 18/VIIIA, the group number represents the number of valence electons. Groups 1/IA-2/IIA have one and two valence electrons, respectively. Groups 13/IIIA to 18/VIIIA have three to eight valence electrons, respectively.
 www.meta-synthesis.com
www.meta-synthesis.com

