That is you got the dianion of the mollykewell cyclo-octatetraene as shown...
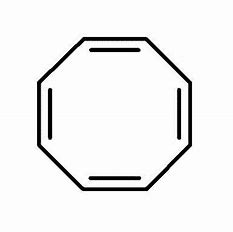
This has EIGHT #pi"-electrons"# and is formally anti-aromatic..(i.e. it does not fulfil the Huckel criteria for aromaticity...) In order to avoid this unfavourable condition..the ring buckles to give a so-called tub shape...
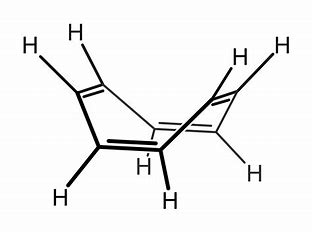
However, the ring can be READILY reduced...by two equiv of alkali metal to give a formal dianion....with #"10# #pi# #"electrons"#...i.e. #C_8H_8^(2-)#...cyclo-octatetraenyl dianion... Such an electronic structure fulfils the Huckel criterion, and this beast is planar and aromatic, and has an extensive coordination chemistry...especially with larger metals...
This is the #"uranium(IV) derivative"#, so-called #"uranocene"#...the uranium analogue of ferrocene..., i.e. #[U(eta^8-C_8H_8)_2]#
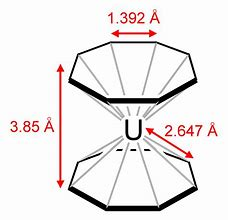
The #C-C# bond lengths are all equivalent, and intermediate between #C-C#, #1.54xx10^-10*m#, and #C=C#, #1.34xx10^-10*m#....




