What element has the highest first ionization energy?
1 Answer
Well, elemental ionization energies INCREASE across a Period, and DECREASE DOWN a Group....
Explanation:
And clearly, this is in relation to the Periodic Table as we face it....
And by these criteria the element with the highest first ionization energy is HELIUM...
And we refer to the transition....
But as chemists, as physical scientists, we SHOULD always interrogate the data...
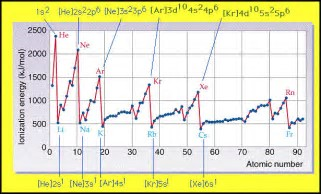
I hope you can see the data here, because I cannot, even with spex on! Do the data support our argument. Why or why not?


