What gas is given off when metals react with acid?
3 Answers
Hydrogen (H2). This was the method used by the first manned-balloon flight using hydrogen (brainchild of Jacques Charles - the namesake for Charles law)
Explanation:
A quarter tonne of sulfuric acid was reacted with half a tonne of iron to fill
https://en.wikipedia.org/wiki/Jacques_Charles#First_hydrogen_balloon
Hydrogen(H2) gas is given of when metals react with an acid.
It depends on the metal, but when you work with Aluminum as an example, the reaction becomes:
#color(blue)("2Al"(s))# #color(blue)(+)# #color(blue)(6"HCl"(aq) -> 2"AlCl"_3(aq))# #color(blue)(+)# #color(blue)(3"H"_2(g))#
Another way to see this is the depiction of the reaction mechanism. At a General Chemistry class, you do not need to know this mechanism, but maybe it'll help to get a "behind-the-scenes" on what happens.
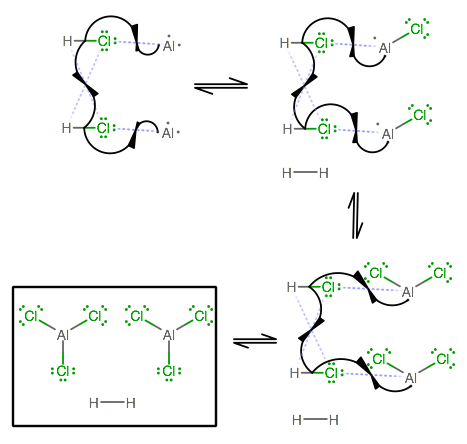
What you should notice is that indeed, two
After three steps, we form two equivalents of
As an interesting aside, if we had two more equivalents of
#color(blue)(2"Al"(s))# #color(blue)(+)# #color(blue)(8"HCl"(aq) -> 2"AlCl"_4^(-)(aq))# #color(blue)(+)# #color(blue)(3"H"_2(g) + "2H"^(+)(aq))#
Since aluminum now has no more valence electrons to donate, it must utilize its empty



