What happens as water freezes?
1 Answer
May 8, 2017
The particle's overall kinetic energy has decreased, causing a stronger attraction between the particles.
Explanation:
Freezing implies the removal of heat of an object.
An object that is cold has particles that are moving really slow.
Afterall, the overall kinetic energy of the particles is equivalent to the object's temperature.
By removing heat, the particles become less "excited" and will collide with other particles much less.
This gives a chance for the attraction between the particles to "grow stronger". With the decrease of particle speed and the increased attraction strength.
This ultimately results in a change in the state of matter from gas -> liquid -> solid.
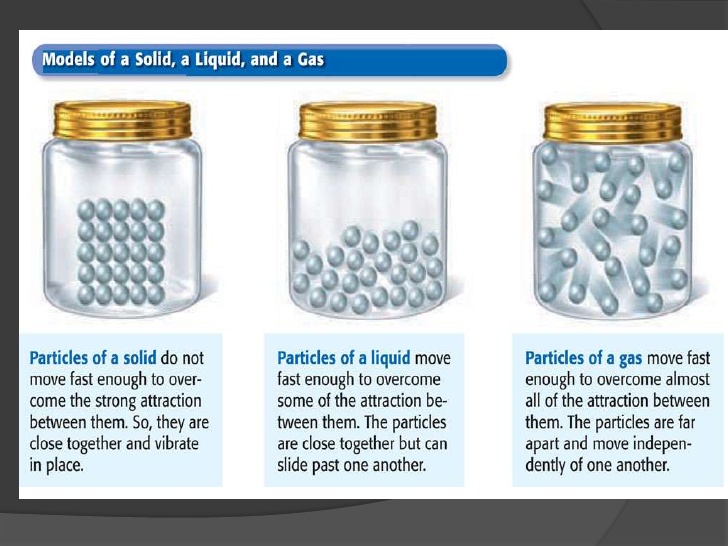
Hope this helps :)

