What happens to the vapor pressure as the surface area of a liquid decreases?
1 Answer
Feb 20, 2016
Nothing happens to the vapour pressure as the surface area increases.
Explanation:
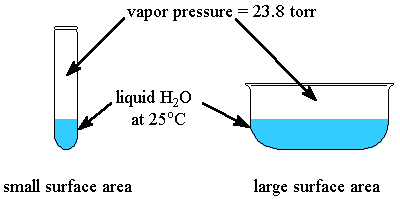
The vapour pressure of a liquid depends only on the temperature and its internal forces of attraction.
Vapour pressure is the pressure exerted by the vapour in a closed container when the rate of evaporation equals the rate of condensation.
When a liquid evaporates in the open, the condensation rate is negligible.
Increasing the surface area increases the evaporation rate, but it has little effect on the condensation rate.
 www.chem.purdue.edu
www.chem.purdue.edu
Hence, water in an open dish evaporates faster than water in a test tube, but the vapour pressure does not change.

