What happens when Group 2A elements form ions?
1 Answer
They lose
Explanation:
As you know, neutral atoms become ions by losing or by gaining electrons.
The number of valence electrons an atom has will determine whether or not it loses electrons to become a **positively charged ion, or cation, or gains electrons to become a *negatively charged ion, or anion.
In this case, elements located in group 2 will always lose electrons to become cations.
More specifically, they will always lose
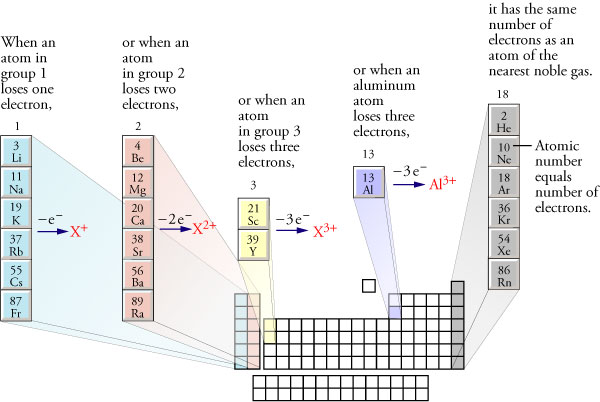
In the case of beryllium, which is located in group 2, period 1, losing
For the other elements, losing

