What happens when I give dilute H2SO4 in 2-Methyl propanol?
1 Answer
Here's what I think.
Explanation:
Dilute sulfuric acid at room temperature
Nothing much will happen that you can see,
The alcohol will dissolve in the aqueous acid and will be in an equilibrium protonation of the alcohol group with hydronium ion.
On long standing at room temperature
The protonation of the
The problem is that the loss of a water molecule generates an unstable primary carbocation.
The generation of the primary carbocation would have a high activation energy and be extremely slow.
However, we have learned that, if a carbocation can become more stable by rearranging, it will do so.
If the rearrangement occurs at the same time as the loss of the leaving group, the activation energy will be lower, and the reaction rate will be faster.
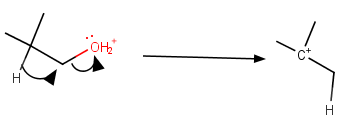 Loss of leaving group
Loss of leaving group
The loss of water with the simultaneous migration of a hydrogen atom generates a more stable tertiary carbocation.
The newly-generated cation can then react with water and form a tertiary alcohol.
The reaction may proceed at a measurable rate at room temperature.

