What happens when the temperature of a solid reaches its melting point?
1 Answer
Jan 8, 2016
The temperature remains constant until all of the solid is melted.
Explanation:
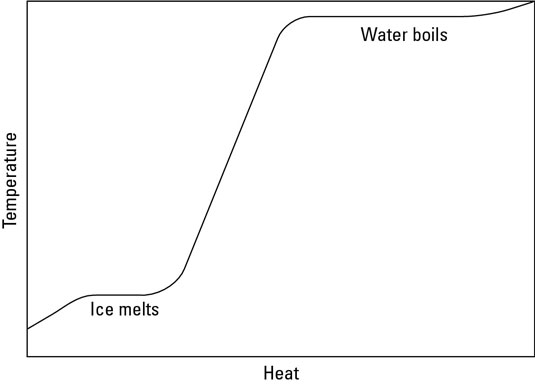
As you can see on the phase change diagram for water, as heat is added, temperature increases, except for a couple of level regions where a phase change takes place; water ice melting, and liquid water boiling. During these phase changes, heat is still being added, but during the phase change, that energy causes the change in phase, and the temperature doesn't rise. Once the phase change is complete, the heat again increases the temperature, until the temperature increase reaches another phase change, where it remains constant again.