What is a buffer solution and how does it work?
1 Answer
A buffer solution is comprised of a weak acid and its conjugate base, such that,
It's very apparent when evaluating a titration curve,
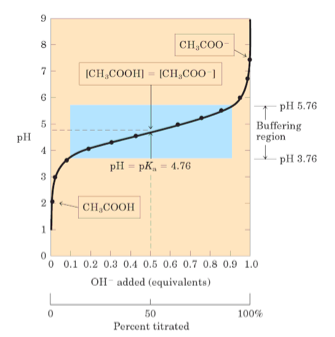
In the buffering region, the rate of

Two equilibria are coexisting, and when one transiently exceeds its equilibrium constant, it readjusts and affects the other equilibrium, that further adjusts.
Hence, the function of a buffer is to stabilize the
A notable example is the bicarbonate buffer system in your blood plasma,
where
Enzymes function at characteristic optimal

