What is a radioactive isotope? Why are they radioactive?
1 Answer
Isotopes of an element are different forms of the same element with the same number of protons and a different number of neutrons. Some of these nuclei, however, might contain too high a proportion of protons or neutrons, which will cause it to be unstable and prone to undergoing radioactive decay in which the nucleus will emit certain forms of radiation in a decay chain until it reaches a stable state. Unstable nuclei will continue to emit radiation and stabilise to lower energy states until the system is stable.
The diagram below shows the correlation between the number of protons and neutrons in the nucleus and the stability of the nucleus:
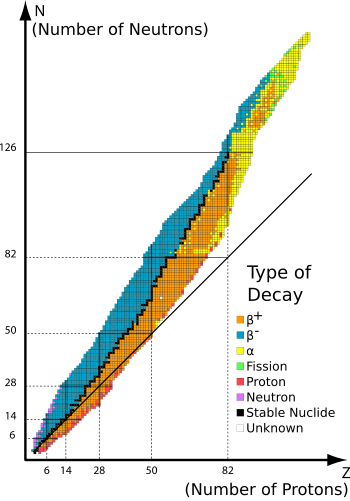
For instance, if the nucleus has too many neutrons, it will perhaps undergo beta minus decay (blue) in which a neutron will turn into a proton, plus an electron antineutrino and an electron. An example is the decay of Carbon-14 into Nitrogen-14, which happens to be a very useful reaction for radiocarbon dating. Likewise, a lower mass radioisotope with an excess of protons will try to return to a more stable state by reducing the number of protons and increasing the number of neutrons, by undergoing beta plus decay (orange).
As for large nuclei, the distance between all of the nucleons is usually too great for the strong nuclear force (which has a very low range) to act over, so it cannot oppose the electrostatic repulsion between the protons in the nucleus and this leads to decay, for example, the emission of an alpha particle (yellow).
And in some cases after other types of decay, a nucleus will possess excess energy leaving it in an excited state; this excess energy is often released in the form of a gamma ray.
The key concept is to remember that radioactive decay is a method for unstable nuclei to release energy, causing a reconfiguration of the structure of the nucleus which brings it to a lower potential energy state.


