What is a rule for determining the mass number of an atom?
1 Answer
Nov 8, 2015
Mass number is the sum of the protons and neutrons in the atomic nucleus.
Explanation:
The mass number (
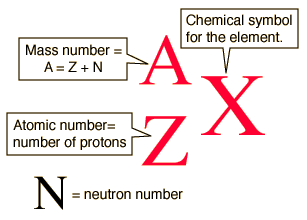 http://hyperphysics.phy-astr.gsu.edu/hbase/nuclear/nucnot.html
http://hyperphysics.phy-astr.gsu.edu/hbase/nuclear/nucnot.html

