pH is just a fancy scale for representing the concentration of #H_3O^+# (hydronium) ions in the solution. To find the concentration of hydronium ions, you just take #10^(-pH)#, which simplifies to #1/10^(pH)#, if you prefer seeing it that way.
As you can see, therefore, substances with smaller pH values will have a #color(red)("larger hydronium ion concentration.")# and we call these #color(red)("acidic")#
Similarly, substances with larger pH values will have #color(blue)("lower hydronium ion concentrations")#, and we call these #color(blue)("basic")#
Our baseline is that a #color(green)("pH of 7 is considered neutral")#. Anything above is basic, anything lower is acidic.
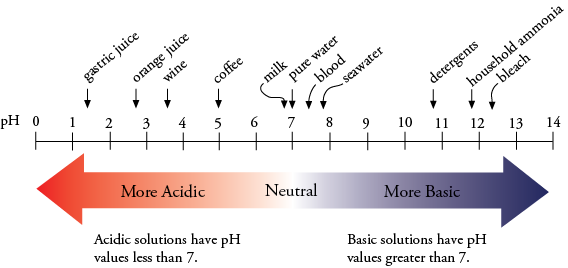
So, bearing all that in mind, a pH of 4 would be considered #color(red)("acidic")#
Hope that helps :)


