What is a understandable explanation of bonding in ammonia?
In terms of polar and non polar molecules
In terms of polar and non polar molecules
1 Answer
Jan 31, 2018
Ammonia is a polar molecule.
Explanation:
Since nitrogen has a much higher electronegativity than hydrogen
This makes the molecule polar.
Have a look at this picture:
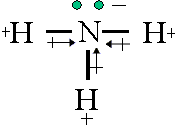
As you can see here, there are lone electrons in the nitrogen atom, so they give the molecule a negative charge on one end, and the positive hydrogen atoms give a positive charge, and thus making the whole molecule polar.

