What is an ion and how is it different from an atom?
2 Answers
Explanation:
A given atom, say
But because there is now a mismatch between NUCLEAR charge and ELECTRONIC charge, an ion results.............. Most elements form ions of different sorts, which may be predicted on a Periodic basis.
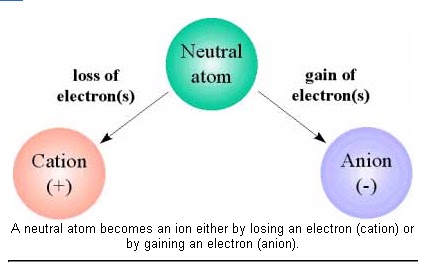
An ion is an atom that has lost or gained one or more electrons.
Explanation:
An atom is neutral when it has equal numbers of protons and electrons.
An ion is an atom that is no longer neutral because it has either lost or gained one or more electrons. When an atom loses one or more electrons, it becomes a positively charged ion, called a cation . When an atom gains one or more electrons, it becomes negatively charged, called an anion .