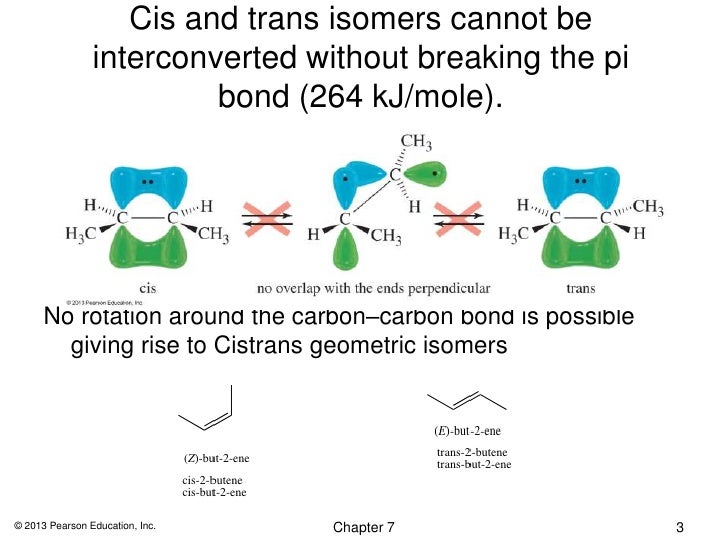
What is "breaking the Pi bond" in alkenes?
1 Answer
Breaking the π bond in an alkene means twisting one end of the bond relative to the other so that the
A π bond in an alkene forms when the
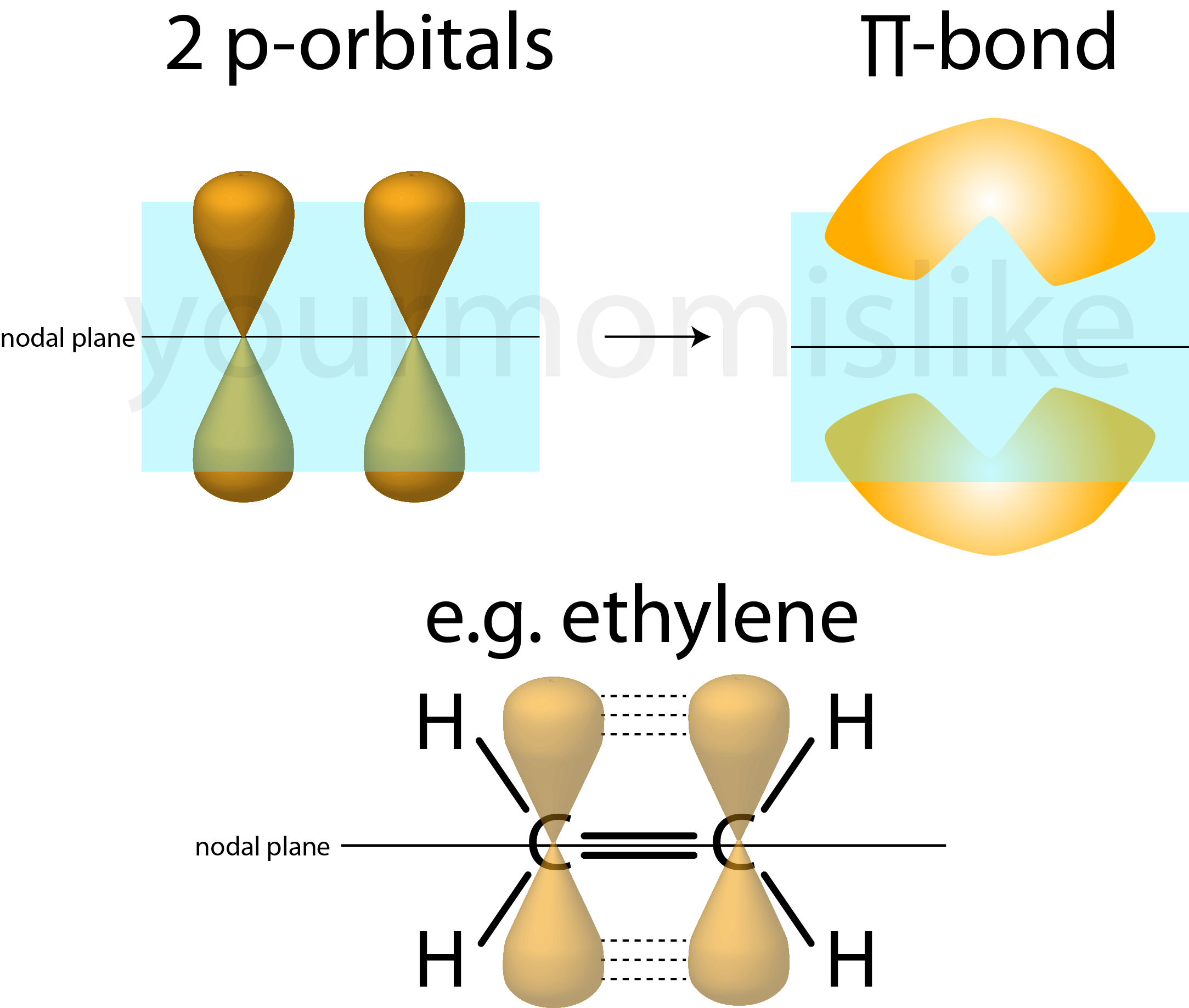
The most stable arrangement is when the two p orbitals are parallel. This gives the most effective overlap.
As you rotate one
When the
Breaking the π bond takes energy, so the most stable geometry of the molecule is planar. You can disturb this arrangement only by adding energy.
The result is that there are cis and trans isomers of but-2-ene. A sample of one of these compounds does not become the other. You would have to break the π bond, and there isn't enough energy available to do that.