What is double bonding?
1 Answer
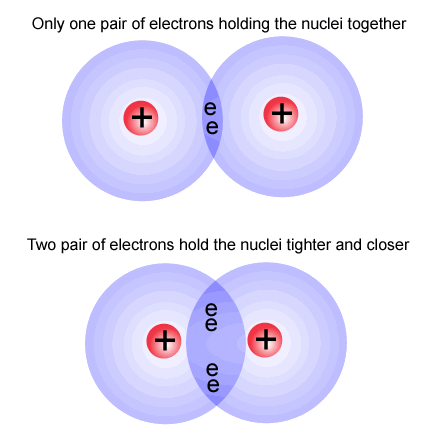
Double bonding occurs when two atoms of non-metal elements share two pairs of electrons, in what is called a double bond. A double bond is composed of two covalent bonds. In a structural formula, a double bond is represented by two parallel lines, each line representing a covalent bond between atoms.
Carbon atoms form double bonds ( C=C ) with each other in many organic compounds, which is why it is so versatile in the formation of organic compounds and biomolecules. The oxygen atoms in an
 http://picornot.com/domain/ibchem.com
http://picornot.com/domain/ibchem.com
As you can see in this diagram the electrons are being shared between two atoms. The top part is a single bond and the bottom part is a double bond.
Image taken from: http://picornot.com/domain/ibchem.com


