What are hydrides?
1 Answer
Aug 24, 2017
Hydrides, or more likely what you mean is metal hydrides, are compounds containing a metal and an
The hydrogen atom has a significantly higher electronegativity than many transition metals, so we can treat the interaction as a complete electron transfer (i.e. ~100% ionic character).
Some example metal hydrides are:
-
#"NaH"# (sodium hydride), used in organic chemistry often to remove an#"H"^(+)# from acetylene for reaction with alkyl halides, a#"C"-"C"# bond-making reaction. -
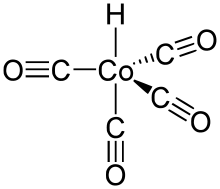
#"HCo"("CO")_4# (tetracarbonylhydridocobalt(I)), a trigonal bipyramidal transition metal complex.
#"LiAlH"_4# (lithium aluminum hydride), a very strong reducing agent used in organic chemistry. It reacts sufficiently with carboxylic acids, amides, and esters, whereas#"NaBH"_4# (sodium borohydride) would be insufficiently reactive.